SINGAPORE, Dec. 23, 2018 /PRNewswire/ — HistoIndex’s AI-based SHG technology has the potential to yield important results from NASH clinical trials. HistoIndex’s SHG technology was featured in Madrigal Pharmaceuticals’ Presidential Plenary presentation during AASLD’s The Liver Meeting® 2018, for its presentation entitled, “In a Placebo Controlled 36 Week Phase 2 Trial, Treatment with MGL-3196 Compared to Placebo Results in Significant Reductions in Hepatic Fat (MRI-PDFF), Liver Enzymes, Fibrosis Biomarkers, Atherogenic Lipids, and Improvement in NASH on Serial Liver Biopsy“.
Liver biopsy is crucial in the precise assessment of fibrosis progression/regression where biopsy-based endpoints are required for U.S. Food and Drug Administration (FDA) approval. Using SHG, analyses can be achieved with a continuous-scale measurement to objectively quantify fibrosis. Such data can be used to inform treatment efficacy as well as primary/secondary endpoint selection in NASH Phase 2B and Phase 3 clinical trials. Says Professor Stephen Harrison, Medical Director of Pinnacle Clinical Research; Visiting Professor of Hepatology, Oxford University and Principal Investigator of the study, “Although the utilization of SHG technology in NASH clinical trials is currently exploratory, it is nonetheless being used in multiple NASH clinical trials for continuous measurements of liver fibrosis. Data generated from the SHG technology can potentially support decision-making for NASH drug development.”
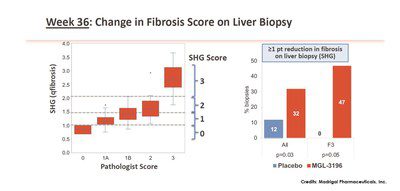
Data from Madrigal’s Phase 2 clinical trial showed that by using SHG, MGL-3196 treated compared with placebo showed a statistically significant 1 point or more reduction in fibrosis score at Week 36 (32% of MGL-3196 treated patients vs. 12% in placebo). Based on pathology score, fibrosis was reduced by 1 point or more in 29% of MGL-3196 treated patients vs. 23% in placebo. Focusing on patients with only F3 fibrosis, it was shown that, using SHG, 47% of the MGL-3196 group had one point or more reduction in fibrosis while none of the placebo group showed the same change. For pharma and biotech companies in NASH drug development, regression in fibrosis is a favourable outcome that potentially demonstrates overall effectiveness of treatments.
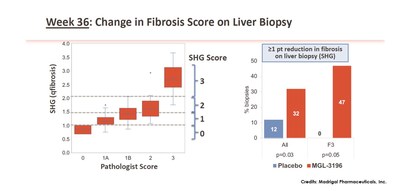
Week 36: Change in Fibrosis Score on Liver Biopsy. From: \”In a Placebo Controlled 36 Week Phase 2 Trial, Treatment with MGL-3196 Compared to Placebo Results in Significant Reductions in Hepatic Fat (MRI-PDFF), Liver Enzymes, Fibrosis Biomarkers, Atherogenic Lipids, and Improvement in NASH on Serial Liver Biopsy\”. Image Credits: Madrigal Pharmaceuticals, Inc.
- Second Harmonic Generation (SHG) microscopy provides automated fully quantitative assessment of fibrosis on liver biopsy slides based on unique architectural features of collagen.
- SHG score was generated and aligned with the pathologist baseline score (baseline, r=0.76), (left panel), blinded to treatment code.
- Using SHG, MGL-3196 treated compared with placebo showed a statistically significant ≥1-point reduction in fibrosis score at Week 36. Based on pathology score, fibrosis was reduced by ≥1 point in 29% of MGL-3196 treated patients vs. 23% in placebo.
HistoIndex’s SHG technology is being employed in about 10 various NASH and Hepatitis B FDA clinical trials, and has assisted many other organizations to identify fibrotic changes in their preclinical and clinicial studies. “The continuous-scale quantification of fibrosis (qFibrosis) from these studies have provided our pharma and biotech partners with the confidence in planning and executing their NASH clinical trials as well as advancing treatments for patients.” – Dr Gideon Ho, Chief Commercial Officer, HistoIndex
ANNEX
SHG and its Role in Fibrosis Assessment
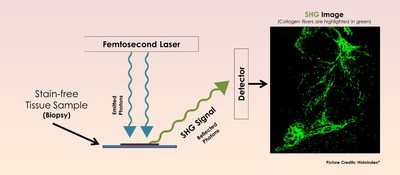
HistoIndex’s proprietary SHG technology resides in a stain-free digital pathology system to characterize and accurately quantify collagen fibers that are an indication of fibrosis.
Collagen fibers, which are unique biological molecules, possess the ability to generate SHG. When a scan begins with an unstained liver tissue sample, the laser produces two photons (tiny light particles), which – upon interaction with the collagen fibers – results in the combination of both photons into a single new photon, with twice the energy and therefore twice the frequency and half the wavelength of the initial photons. This is known as SHG, and the detection of SHG signals lead to a high-resolution imaging of collagen fibers within the tissue.
Distinguishing the morphological changes in the collagen fibers will enable pharmaceutical companies to determine the best treatment for the disease. And when paired with an image analysis algorithm, the system characterizes the collagen fibers and subsequently, generates a fully quantitative and accurate analysis that will aid in the stratification of liver fibrosis and evaluation of treatment efficacy.
About NASH
A chronic and prevalent liver condition that plagues populations battling obesity and diabetes, NASH is in the waitlist for an effective treatment. Trends highlighted by a study indicate that the prevalence of NASH is expected to increase by 63% between 2015 and 2030 (Chris Estes, Hepatology 2018). Multiple clinical trials are in the race towards treatments that could possibly decrease liver fat, inflammation or ballooning, while others aim to reduce fibrosis. If it progresses, fibrosis can lead to liver cirrhosis or liver cancer.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/histoindexs-second-harmonic-generation-shg-technology-a-potentially-important-tool-used-in-madrigals-phase-2-nonalcoholic-steatohepatitis-nash-clinical-trial-300770548.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/histoindexs-second-harmonic-generation-shg-technology-a-potentially-important-tool-used-in-madrigals-phase-2-nonalcoholic-steatohepatitis-nash-clinical-trial-300770548.html



Facebook Comments