A new scientific paper calls for strict adherence to the scientific definition, ensuring improper use of the term doesn’t mislead consumers or limit advancements in the emerging field of microbiome science.
Authored by Dr. Gregor Reid, Raja Dhir, and Dr. Azza A. Gadir
LOS ANGELES, March 14, 2019 /PRNewswire/ — Today, Dr. Gregor Reid, the scientist who chaired the United Nations/World Health Organization Expert Panel that authored the definition of ‘probiotics’ in 20011, published a new paper in Frontiers in Microbiology: “Probiotics: Reiterating What They Are and What They Are Not”, to reestablish and clarify the term.
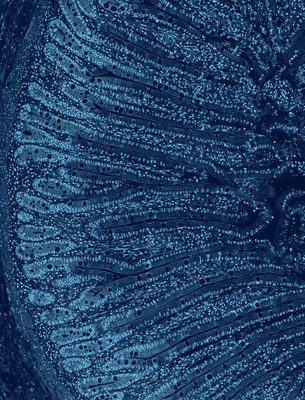
Section of the human small intestine (jejunum). H&E stain. When assessing probiotics, the functional impact on the human gut is as important as metagenomic sequencing to establish definitive clinical outcomes. Photo credit is: Visuals Unlimited, Inc./Dr. Gladden Willis
Marketing and media misuse of the term ‘probiotics’, and even misunderstanding within the scientific community, has contributed to growing global skepticism about the legitimacy of microbial therapies and the associated probiotic applications to improve human health.
Reid authored this paper in an effort to ensure hype doesn’t limit scientific and medical advancements. “Too many microbiome studies oversell limited findings or make broad generalizations that attribute the results of a single study on a single product across our entire field. Adherence by scientists, publications, and reporters to precise definitions and guidelines will ensure more accurate communications,” said Reid. “Stewardship of the term ‘probiotic’ is critical to curb its misuse and misattribution and to protect the future of a field that has incredible promise.”
Summary of Paper
Within the field of microbiome science, the rate of discovery of novel organisms with potentially therapeutic benefit is progressing rapidly and gaining prominence. More than ever, it is imperative that guidelines are followed to determine the validity of a probiotic. As indicated in the original FAO/WHO (2002) report1, there are certain expectations required to call an organism ‘probiotic’. These have been further clarified in 20142, and must include:
- That microbes be alive in an adequate number when administered.
- Strains must be identified genetically, classified using the latest terminology, and designated by numbers, letters, or names.
- Appropriately sized and designed studies must be performed to designate a strain as probiotic and use the strain(s) on the host to which the probiotics are intended (human, livestock, companion animal, etc).
- Strains shown to confer a benefit for one condition may not be probiotic for another application.
- Strains that are probiotic for humans but are being used in animal studies should be clearly designated as human probiotics under experimental testing.
For the complete paper, visit https://www.frontiersin.org/articles/10.3389/fmicb.2019.00424/full
As part of the authors’ commitment to further transparency and accountability in science communication, they have included their conflict of interest statement below. They encourage the scientific community to do the same on press releases.
Raja Dhir is a Co-Founder of Seed Health, Inc., a biotechnology company developing microbial therapies not discussed in this paper. Gregor Reid is a scientific advisor to Seed. Azza A.Gadir is involved in research and development at Seed, and is developing intellectual property related to microbial regulation of immune mechanisms underlying food allergies, which is not discussed in this paper.
____________
References
1 FAO/WHO. (2002). Guidelines for the evaluation of probiotics in food. http://www.fao.org/food/food-safety-quality/a-z-index/probiotics/en/
2 Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D., Pot, B., and Morelli, L. et al. (2014). The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotics. Nat. Reviews Gastroenterol. Hepatol. 11(8):506-14.
Authors
Dr. Gregor Reid, BSc (Hons), PhD, MBA, ARM CCM, Dr HS, FCAHS, FRSC is the Director of the Canadian R&D Centre for Human Microbiome and Probiotics at Lawson Health Research Institute, an inductee into the Royal Society of Canada, and previously served as the President of the International Scientific Association of Probiotics and Prebiotics (ISAPP). He is perhaps best known as the Chair of the United Nations World Health Organization Expert Panel on Probiotics, leading the group that authored the globally accepted definition of ‘probiotics’. Dr. Reid has authored over 520 peer-reviewed papers published in scientific and medical journals, has been a reviewer for 48 international agencies (including the National Institute of Health) and 112 scientific journals; he has been awarded 28 patents and cited over 27,000 times. He also serves as Chief Scientist to Seed.
*
Dr. Azza A. Gadir, PhD completed her postdoctoral training in the laboratory of Dr. Talal Chatila at Harvard Medical School/Boston Children’s Hospital, where her published research focuses on the immunological mechanisms that underlie food allergy. She is specifically interested in understanding the role of the gut microbiome in conferring protection to diseases early in life. For this work, she is co-inventor of a patent for microbial consortia that can reduce and/or eliminate food allergy and has collaborated with industry partners to accelerate the discovery of microbiome-related immunotherapies for food allergy. She is Director of R+D at Seed.
*
Raja Dhir is a life sciences entrepreneur and Co-Founder of Seed, where he leads R+D, academic collaborations, technology development, clinical trial design, supply chain, and intellectual property strategy. Raja serves on the Editorial Board for the scientific journal, Microbiome. He is a member of the Microbiome Think Tank at Mass. General Hospital (MGH) and is a member of the Advisory Committee for the International Scientific Association of Probiotics and Prebiotics (ISAPP).
About Seed®
Seed is a life science and consumer health company pioneering the inquiry and application of microbiome science to improve human and planetary health. In collaboration with leading scientists and a global network of partners and experts in biofermentation, stabilization and testing, Seed is setting a new standard in bacteria. Its environmental R+D arm, SeedLabs, also develops novel applications for bacteria to solve some of our biggest ecological challenges.
Contact:
Erin Allweiss
seed@thenumber29.com
202-446-8265
Photo – https://mma.prnewswire.com/media/834476/Seed_Frontier_cover.jpg